This site is intended for US healthcare professionals only.
- Prescribing Information
Oral, once-weekly XPOVIO dosing
Recommended administration schedule1
![Graphic shows the once weekly dosing schedule of XVd (XPOVIO [selinexor] + bortezomib & dexamethasone) for multiple myeloma.](/_nuxt/img/4.1-pill-chart.f1237ec.png)
![Graphic shows the once weekly dosing schedule of XVd (XPOVIO [selinexor] + bortezomib & dexamethasone) for multiple myeloma.](/_nuxt/img/4-1-recommended-admin-schedule-mb-2x.f2a6c99.png)
XPOVIO 100 mg is taken orally once weekly on day 1 of each week until disease progression or unacceptable toxicity in combination with1:
- Bortezomib 1.3 mg/m2 administered subcutaneously once weekly on day 1 of each week for 4 weeks, followed by 1 week off
- Dexamethasone 20 mg taken orally twice weekly on days 1 and 2 of each week
For additional information regarding the dosing and administration of bortezomib or dexamethasone, refer to the prescribing information for each.
Before starting therapy and during treatment with XPOVIO + Vd, provide 2 antiemetics1,2
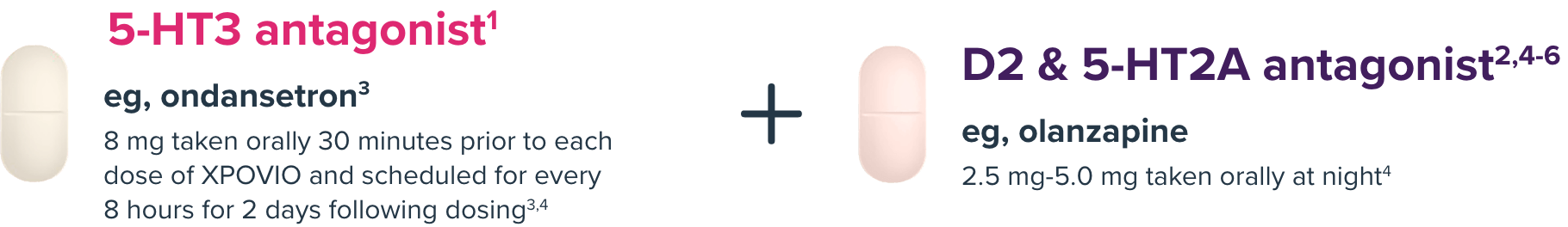

NK-1 antagonists (eg, aprepritant or rolapitant7,8) can be used in place of a D2/5-HT2A antagonist.2,5
Antiemetics such as NK-1 inhibitors and olanzapine can be reduced or stopped after 8 weeks if patients are tolerating selinexor.2
For reference purposes only. All treatment decisions must be individualized and made solely at the discretion of the healthcare professional.
Considerations when starting a patient on XPOVIO + Vd1,2,9-11


Recommended monitoring and management for adverse reactions1
Fluid intake
Ensure patients maintain adequate fluid and caloric intake throughout treatment
IV hydration
Consider IV hydration and replace electrolytes as clinically indicated
Monitor CBC
Monitor CBC with differential, standard blood chemistries, body weight, nutritional status, and volume status at baseline and during treatment as clinically indicated
Thrombocytopenia
Monitor platelet counts at baseline and throughout treatment. Monitor more frequently during the first 3 months of treatment. Institute platelet transfusion and/or other treatments as clinically indicated. Monitor patients for signs and symptoms of bleeding and evaluate promptly. Interrupt, reduce dose, or permanently discontinue based on severity of adverse reaction.
Neutropenia
Obtain white blood cell counts with differential at baseline and throughout treatment. Monitor more frequently during the first 3 months of treatment. Monitor patients for signs and symptoms of concomitant infection and evaluate promptly. Consider supportive measures, including antimicrobials and growth factors (eg, G-CSF). Interrupt, reduce dose, or permanently discontinue based on severity of adverse reaction.
Hyponatremia
Monitor sodium level at baseline and throughout treatment. Monitor more frequently during the first 2 months of treatment. Correct sodium levels for concurrent hyperglycemia (serum glucose >150 mg/dL) and high serum paraprotein levels. Assess hydration status and manage hyponatremia per clinical guidelines, including intravenous saline and/or salt tablets as appropriate and dietary review. Interrupt, reduce dose, or permanently discontinue based on severity of the adverse reaction.
Please see the recommended dosage modification guidelines and additional considerations in the full Prescribing Information.
Additional dosing information1
- Each XPOVIO dose should be taken at approximately the same time of day
- Each tablet should be swallowed whole with water
- Do not break, chew, crush, or divide the tablets
- If a dose of XPOVIO is missed or delayed, instruct patients to take their next dose at the next regularly scheduled time
- If a patient vomits a dose of XPOVIO, the patient should not repeat the dose and should take the next dose on the next regularly scheduled day
Abbreviations: CBC, complete blood count; G-CSF, granulocyte colony-stimulating factor; IV, intravenous; Vd, bortezomib and dexamethasone; XVd, selinexor, bortezomib, and dexamethasone.
References: 1. XPOVIO (selinexor) [prescribing information]. Newton, MA. Karyopharm Therapeutics, Inc. 2. Gavriatopoulou M, Chari A, Chen C, et al. Integrated safety profile of selinexor in multiple myeloma: experience from 437 patients enrolled in clinical trials. Leukemia. 2020;34(9):2430-2440. doi:10.1038/s41375-020-0756-6 3. Zofran. Prescribing information. GlaxoSmithKline; 2016. 4. Chari A, Florendo E, Mancia IS, et al. Optimal supportive care with selinexor improves outcomes in patients with relapsed/refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2021;21(12):e975-e984. doi:10.1016/j.clml.2021.07.014 5. Mikhael J, Belhadj-Merzoug K, Hulin C, et al. A phase 2 study of isatuximab monotherapy in patients with multiple myeloma who are refractory to daratumumab. Blood Cancer J. 2021;11(5):89. doi:10.1038/s41408-021-00478-4 6. Saudemont G, Prod’Homme C, Da Silva A, et al. The use of olanzapine as an antiemetic in palliative medicine: a systematic review of the literature. BMC Palliat Care. 2020;19:56. doi:10.1186/s12904-020-00559-4 7. Emend. Prescribing information. Merck & Co., Inc; 2022. 8. Varubi. Prescribing information. TerSera Therapeutics LLC; 2017. 9. Data on file. Karyopharm Therapeutics Inc. 2021. 10. Colson K. Treatment-related symptom management in patients with multiple myeloma: a review. Support Care Cancer. 2015;23(5):1431-1445. doi:10.1007/s00520-014-2552-1 11. Kurtin S. Living with multiple myeloma: a continuum-based approach to cancer survivorship. Semin Oncol Nurs. 2017;33(3):348-361. doi:10.1016/j.soncn.2017.05.009 12. Data on file [1]. Karyopharm Therapeutics Inc. 2021. 13. Data on file [2]. Karyopharm Therapeutics Inc. 2021.
INDICATION
XPOVIO® (selinexor) is a prescription medicine approved in combination with bortezomib and dexamethasone (XVd) to treat adult patients with multiple myeloma who have received at least one prior therapy.
IMPORTANT SAFETY INFORMATION
Thrombocytopenia: XPOVIO can cause life-threatening thrombocytopenia, potentially leading to hemorrhage. Thrombocytopenia was reported in patients with multiple myeloma.
Thrombocytopenia is the leading cause of dosage modifications. Monitor platelet counts at baseline and throughout treatment. Monitor more frequently during the first 3 months of treatment. Monitor patients for signs and symptoms of bleeding. Interrupt, reduce dose, or permanently discontinue based on severity of adverse reaction.
Neutropenia: XPOVIO can cause life-threatening neutropenia, potentially increasing the risk of infection.
Monitor more frequently during the first 3 months of treatment. Consider supportive measures, including antimicrobials and growth factors (e.g., G-CSF). Interrupt, reduce dose, or permanently discontinue based on severity of adverse reaction.
Gastrointestinal Toxicity: XPOVIO can cause severe gastrointestinal toxicities in patients.
Nausea/Vomiting/Diarrhea: Provide prophylactic antiemetics or treatment as needed.
Anorexia/Weight Loss: Monitor weight, nutritional status, and volume status at baseline and throughout treatment and provide nutritional support, fluids, and electrolyte repletion as clinically indicated.
Hyponatremia: XPOVIO can cause severe or life-threatening hyponatremia.
Monitor sodium level at baseline and throughout treatment.
Serious Infection: XPOVIO can cause serious and fatal infections. Atypical infections reported after taking XPOVIO include, but are not limited to, fungal pneumonia and herpesvirus infection.
Neurological Toxicity: XPOVIO can cause life-threatening neurological toxicities.
Coadministration of XPOVIO with other products that cause dizziness or mental status changes may increase the risk of neurological toxicity.
Advise patients to refrain from driving and engaging in hazardous occupations or activities, until the neurological toxicity fully resolves. Institute fall precautions as appropriate.
Embryo-Fetal Toxicity: XPOVIO can cause fetal harm when administered to a pregnant woman.
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential and males with a female partner of reproductive potential to use effective contraception during treatment with XPOVIO and for 1 week after the last dose.
Cataracts: New onset or exacerbation of cataract has occurred during treatment with XPOVIO. The incidence of new onset or worsening cataract requiring clinical intervention was reported.
ADVERSE REACTIONS
The most common adverse reactions (ARs) (≥20%) in patients with multiple myeloma who received XVd were fatigue, nausea, decreased appetite, diarrhea, peripheral neuropathy, upper respiratory tract infection, decreased weight, cataract, and vomiting.
Grade 3-4 laboratory abnormalities (≥10%) were thrombocytopenia, lymphopenia, hypophosphatemia, anemia, hyponatremia and neutropenia.
Fatal ARs occurred in 6% of patients within 30 days of last treatment. Serious ARs occurred in 52% of patients. Treatment discontinuation rate due to ARs was 19%. The most frequent ARs requiring permanent discontinuation in >2% of patients included fatigue, nausea, thrombocytopenia, decreased appetite, peripheral neuropathy and vomiting. Adverse reactions led to XPOVIO dose interruption in 83% of patients and dose reduction in 64% of patients.
USE IN SPECIFIC POPULATIONS
No overall difference in effectiveness of XPOVIO was observed in patients >65 years old when compared with younger patients. Patients ≥65 years old had a higher incidence of discontinuation due to an adverse reaction (AR) and a higher incidence of serious ARs than younger patients.
The effect of end-stage renal disease (CLCR <15 mL/min) or hemodialysis on XPOVIO pharmacokinetics is unknown.
Please see full Prescribing Information.
To report SUSPECTED ADVERSE REACTIONS, contact Karyopharm Therapeutics Inc. at 1-888-209-9326 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
© 2023 Karyopharm Therapeutics Inc.
X


![Graphic shows the once weekly dosing schedule of XVd (XPOVIO [selinexor] + bortezomib & dexamethasone) for multiple myeloma.](/_nuxt/img/4-1-getting-patients-started-guide-2x.d50467f.jpg)


